Calcuating Cancer Risk Using Oral Slope Factor for Continuous Lifetime Exposure From Birth
Once you have your estimated exposure doses and adjusted air concentrations, you will use health guidelines and cancer risk values to estimate the potential non-cancer health effects and cancer risks, respectively, for each contaminant and exposure pathway.
Selecting Appropriate Health Guidelines and Cancer Risk Values
Before performing the HQ and CR calculations, you will need to determine the most appropriate health guidelines or cancer risk values, respectively, to use for evaluating your site-specific exposure doses or adjusted air concentrations. Important: PHAST will make this selection for you, but it is important for health assessors to understand ATSDR's selection process. Different health guidelines are available for exposure routes (ingestion, inhalation), exposure durations (acute, subchronic/intermediate, and chronic), and health endpoints (carcinogenic, non-carcinogenic).
Health guidelines (used to evaluate non-cancer health effects) (see descriptions in this table) and cancer risk values (used to estimate cancer risks) (see descriptions in this table) are derived from data in the epidemiologic and toxicologic literature with appropriate uncertainty or safety factors applied to ensure they are set at levels below those that could result in harmful health effects. The values do not represent thresholds of toxicity.
Common Health Guidelines (Non-Cancer) Used by ATSDR
| Health Guidelines | Definition |
|---|---|
| ATSDR-Developed Minimal Risk Levels (MRLs) |
|
| EPA-Derived Reference Doses (RfDs) |
|
| EPA-Derived Reference Concentrations (RfCs) |
|
Common Cancer Risk Values Used by ATSDR
| EPA-Derived Cancer Risk Values | Definition |
|---|---|
| Oral Cancer Slope Factors (CSFs) |
|
| Inhalation Unit Risks (IURs) |
|
Again, remember that PHAST will select the most appropriate health guidelines to evaluate non-cancer effects and cancer risk values to evaluate cancer effects for your scenarios. However, it is important for health assessors to understand ATSDR's selection process. The following considerations apply when selecting the most appropriate health guidelines and cancer risk values:
- Exposure route. If contaminant-specific health guidelines or cancer risk values are not available for the exposure route of concern at a site, depending on specific scenarios, health guidelines or cancer risk values developed for other exposure routes could be used. For example, since health guidelines and cancer risk values are not available for dermal contact, ATSDR uses oral minimal risk levels (MRLs) and cancer slope factors (CSFs) for ingestion exposures to evaluate dermal exposures in PHAST. When PHAST does not have available health guidelines or cancer risk values, health assessors will further evaluate those contaminants in the in-depth toxicological effects analysis. If health assessors identified health guidelines and cancer risk values from other sources (see box) for other exposure routes, before using this approach, consult with a toxicologist and the ADS group and consider the impact of extrapolating from one route of exposure to another in these cases. Exercise care when drawing conclusions from these types of comparisons, and be sure to note you used a substitute approach and indicate it could result in lower confidence in the associated results.
Estimating Hazard Quotients
The hazard quotient (HQ) is calculated to evaluate the potential for non-cancer health hazards to occur from exposure to a contaminant with available non-cancer health guidelines (MRLs, RfDs, RfCs).
When you have exposure doses, you typically obtain the HQ by dividing the duration-specific (acute, intermediate, or chronic) exposure dose by the non-cancer health guideline for the same duration MRL or RfD for your exposure groups of interest. When you have an air concentration, you obtain the HQ by dividing the duration-specific exposure concentration by the MRL or RfC. The box below contains the basic formula for calculating HQs using exposure doses or adjusted air concentrations.
ATSDR calculates CTE and RME CRs, depending on what is appropriate for the site-specific scenario. PHAST will calculate these CRs for you using the approach that follows. For children, CRs are derived for a combined child: CTE (12 years) and RME (21 years) at a given residence. For the CTE child CR, the combined child is the sum of the cancer risks for each age group for the first 12 years of exposure only. The RME CR for the combined child is derived by summing all the cancer risks for each age group from birth to < 21 years. The adult CR assumes living at the residence for 12 (CTE) or 33 (RME) years. PHAST can calculate CRs for contaminants with available cancer risk values.
Once you have your CR, see if it is greater than 1.0E-06. Health assessors retain those contaminants with CRs greater than 1.0E-06 and conduct an in-depth toxicological effects analysis.
The example below uses the cancer risk equation formula to calculate a cancer risk.
Considering Cancer Risk for Mutagens
Another important concept is carcinogens that have a mutagenic mode of action (MOA). Children are more susceptible to cancer and tumor development if exposed to carcinogens with a mutagenic MOA. To account for this increased susceptibility, ATSDR applies age-dependent adjustment factors (ADAFs) to the CR equation for these contaminants. The ADAF-adjusted cancer risk equation is in the box below.
Cancer risk is calculated differently for one contaminant: trichloroethylene (TCE). Cancer risk calculations for TCE incorporate three cancer types (non-Hodgkin's lymphoma, kidney and liver cancer). Because TCE is only considered to be mutagenic for kidney cancer, ADAFs are only applied to the kidney cancer portion of the cancer slope factor. PHAST automatically incorporates these differences for TCE into its cancer risks calculations.
The example below uses the ADAF-adjusted cancer risk formula shown above to calculate cancer risks for a mutagenic carcinogen.
Scenario: Let's use a new drinking water exposure example to examine how to estimate potential mutagenic carcinogenic risks. For this example, a family had chronic daily residential exposure to contaminated drinking water with an EPC of 0.00015 milligrams per liter (mg/L) for 1,2,3-trichloropropane—a mutagen with an oral CSF of 30 (mg/kg/day)-1. You have no specific information on the residents.
Let's do one calculation together to estimate the RME cancer risk for the 2 to < 6 years age group. First, we need to calculate the RME exposure dose using the default intake rate of 0.977 mg/L and default body weight of 17.4 kg for this age group. We will use the drinking water ingestion equation to calculate the RME dose as follows:
RME Dose = (C x IR x EF) / BW
RME Dose = (0.00015 mg/L x 0.977 L/day x 1) / 17.4 kg
RME Dose = 8.4E-06 mg/kg/day
Now we will use the ADAF-adjusted cancer risk formula to calculate the RME cancer risk for this one child age group. Remember: In PHAST, ATSDR calculates an RME CR for the combined child by summing all CRs for each age group from birth to < 21 years. The calculation we are doing here represents just one CR that would go into the combined child CR sum. PHAST will do these calculations for you, but it's important to understand how the calculations work.
ADAF-adjusted CR = (D × CSF) × (ED / LY) × ADAF
ADAF-adjusted CR = (8.4E-06 mg/kg/day x 30 (mg/kg/day)-1) x (4 / 78) x 3
ADAF-adjusted CR = 3.9E-5
Conclusion = Cancer risk is greater than 1.0E-6. The health assessor should conduct an in-depth toxicological effects analysis.
See the PHAST results table for this scenario below:
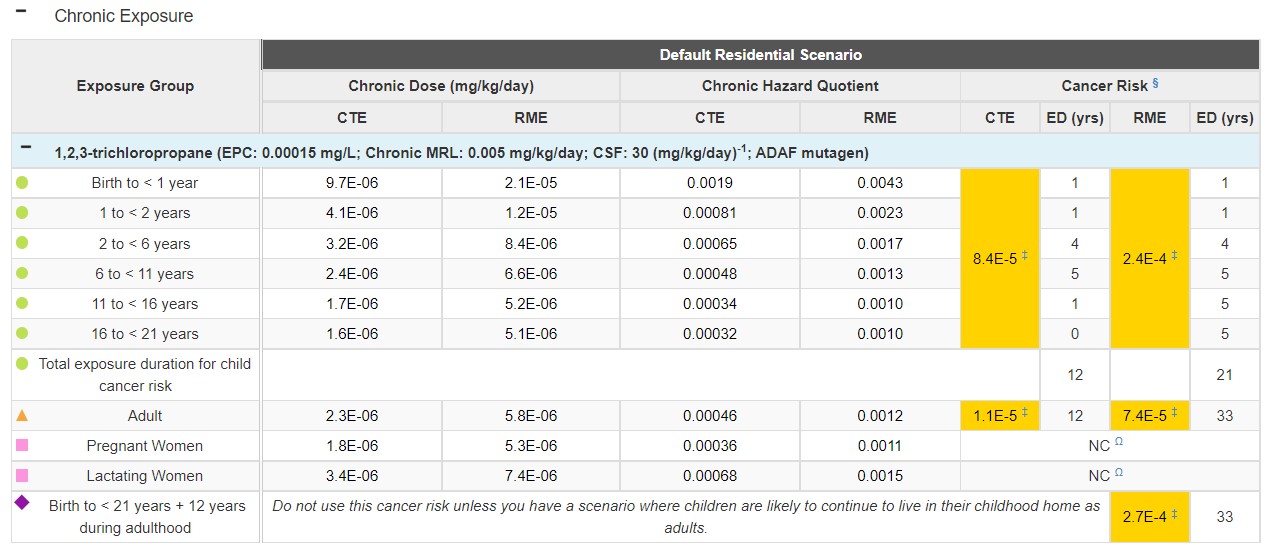
Source: https://www.atsdr.cdc.gov/pha-guidance/conducting_scientific_evaluations/epcs_and_exposure_calculations/hazardquotients_cancerrisk.html